CFI™ (Critical Fluid Inactivation) – Pathogen Reduction for Blood Products, Protein Therapeutics and Medical Devices such as PPEs
For the physical inactivation of enveloped and non-enveloped viruses with no residual contamination and negligible denaturation of proteins and enzymes
The Problem
The worldwide AIDS epidemic, the periodic emergence of Ebola and SARS, and the recent outbreaks of potentially pandemic strains of influenza such as H5N1 have highlighted a persistent concern in the health-care community – the need for effective pathogen inactivation and removal techniques for human blood plasma and plasma-derived products.
A number of approaches have been employed for the inactivation or removal of viruses in human plasma and therapeutic proteins derived from human plasma, including heating or pasteurization; solvent-detergent technique; Ultra Violet (UV) irradiation; and chemical inactivation. Current approaches are not always effective against a wide spectrum of human and animal viruses, are sometimes encumbered by process-specific deficiencies, and often result in denaturation of the biologics that they are designed to protect.
The CFI™ Solution
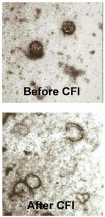 CFI™ works by first permeating and inflating the pathogen particles with a selected supercritical, critical or near-critical fluid with or without polar cosolvents [SuperFluids™ or SFS]. The ultra-low interfacial tension of SuperFluids™ allows facile penetration into microporous structures. As such, SuperFluids™ can readily penetrate and inflate viral particles.
CFI™ works by first permeating and inflating the pathogen particles with a selected supercritical, critical or near-critical fluid with or without polar cosolvents [SuperFluids™ or SFS]. The ultra-low interfacial tension of SuperFluids™ allows facile penetration into microporous structures. As such, SuperFluids™ can readily penetrate and inflate viral particles.
The overfilled particles are then decompressed. The dense-phase fluid rapidly changes into a gaseous state, rupturing the pathogen particles at their weakest points – very much like the embolic disruption of the eardrums of a scuba diver who surfaces too rapidly – and becomes inactive.
The now gaseous SFS leaves the protein-rich substrate with no toxic residuals to be removed.
CFI™ Benefits/Advantages
- CFI™ does not damage proteins and enzymes since it is purely physical and does not involve the use of chemicals, heat or irradiation.
- A key feature of this technology is that proteins and enzymes are negligibly impacted and remain biologically active.
- Based on purely physical principles, CFI does not rely on potentially toxic organic solvents, DNA/RNA modifying chemicals, denaturing heat or irradiation techniques.
- CFI™ is a closed system, fluid-liquid/solid contacting technique that is readily scalable and amenable to batch or continuous flow operations.
CFI™ Products and Services
Aphios manufactures and sells standardized CFI™ units for processing units of biologics that will be fully automated, mobile and about the size of a washing machine. These units have the capability to process about 100 units of plasma per day and reduce pathogen levels by > 4 logs per mL with > 90% retention in biological activity. Customized CFI™ units have the capability to process 1,000 to 10,000 liters per day with similar pathogen reduction and biological retention levels.
Aphios licenses include CFI™ technology and equipment for customers. Licensing agreements include royalties on a per product use basis, CFI™ equipment and raw materials (SuperFluids™), and service and maintenance contracts. CFI™ equipment are standardized for certain operations such as blood plasma process and PPEs, and customized for high throughput manufacturing of pooled plasma, fetal bovine serum, immunoglobulins, monoclonal antibodies and recombinant therapeutics.
Aphios collaborates with strategic corporate partners and medical institutions to implement a pathogen inactivation technology that is purely physical and effective without product denaturation for your pathogen reduction applications.