For intravenous and/or topical administration of hydrophilic molecules including siRNA and hydrophobic anticancer drugs
Conventional Liposomes and Manufacturing Challenges
Phospholipid liposomes are small vesicles comprised of single or multiple lipid bilayers. Liposomes are non-toxic, non-antigenic and biodegradable since they have the molecular characteristics of mammalian cell membranes.
Most conventional liposomes are manufactured by organic solvents utilizing rotary evaporation techniques or homogenization that utilizes high pressure, multiple recycle techniques. These methods often result in denaturation of the encapsulated drug by excessive heat or organic solvents, contain residual organic solvents and are not readily scalable.
Phospholipid nanosomes (small, uniform liposomes) were developed to overcome some of the limitations associated with the conventional manufacturing of liposomes.
A Solution – The CFN Process
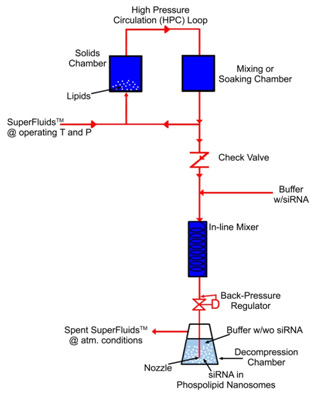 In the CFN (critical fluid nanosomes) process, supercritical, critical or near-critical fluids with or without polar cosolvents [SuperFluids™ (SFS)] at appropriate conditions of pressure and temperature are utilized to solvate phospholipids, cholesterol, and other nanosomal raw materials.
In the CFN (critical fluid nanosomes) process, supercritical, critical or near-critical fluids with or without polar cosolvents [SuperFluids™ (SFS)] at appropriate conditions of pressure and temperature are utilized to solvate phospholipids, cholesterol, and other nanosomal raw materials.
After a specific residence time, the resulting mixture is decompressed via a backpressure regulator (valve) though a dip tube with a nozzle into a decompression chamber that contains phosphate-buffered saline or other biocompatible solution.
Bubbles will form at the injection nozzle tip because of SFS depressurization and phase-conversion into a gas, and the solvated phospholipids will deposit out at the phase boundary of the aqueous bubble.
As the bubbles detach from the nozzle into the aqueous solution, they rupture, causing bilayers of phospholipids to peel off, thereby encapsulating solute molecules and spontaneously sealing themselves to form phospholipid nanosomes.
This CFN injection technique is ideally suited for the nanosomal encapsulation of recombinant proteins for enhanced drug delivery, DNA used in gene therapy, and siRNA for improved drug delivery. In other CFN techniques, upon decompression and separation of the SuperFluids™, phospholipid nanosomes are formed to improve the delivery and therapeutic efficacy of siRNA and poorly water-soluble drugs while reducing toxicity [US Patents].
CFN Benefits/Advantages
- Integrity of the nanosomes and encapsulated therapeutics resulting from not using organic solvents
- Scalability in that the CFN manufacturing processes are essentially liquid-liquid type unit operations
- Process flexibility in that there are several degrees of freedom such as nozzle type and size, operating pressure and temperature, SuperFluids type and cosolvent concentration as well as CFN technique to customize and optimize nanosome characteristics
- Formulation stability and end product sterility resulting from operating in an oxygen-free environment combined with the physical disruption/inactivation of viruses and other pathogens as a result of decompression
- No excessive heat that can damage thermally labile proteins and enzymes, unlike high-pressure homogenization; CFN instead produces a Joule-Thompson cooling effect
- Negligible shear which will be beneficial for shear sensitive therapeutic proteins and nucleic acids
- Lower pressure requirements as compared to high-pressure homogenization resulting in reduced equipment wear and operating costs
- Higher speed of processing because the CFN processes are single-pass ones not requiring the multiple passes of high-pressure homogenization
- Ability to operate continuously – the CFN processes can be readily adapted to continuous flow operation because they are single-pass ones in which the working solvent can be readily separated, recovered and reused
We utilize five different critical fluid nanosomes (CFN) techniques for the nanoencapsulation of therapeutics – injection, co-injection, decompression, evaporation, and co-encapsulation.