Clinically tested to be significantly effective for treating nausea from chemotherapy without any adverse side effects [US Patent, 2013]
Nausea Remains a Significant Problem with Current Anti-CINV Drugs
Despite the widespread use of the 5-HT3 receptor antagonist anti-emetics such as Palonosetron (Aloxi®; Eisai), Ondansetron (Zofran®; GSK), Granisetron (Kytril®; Roche Pharmaceuticals), and Dolasetron (Anzemet®; Aventis), CINV continues to be reported by up to 70% of adult patients receiving highly emetogenic chemotherapy agents and 58% of school-age and adolescent-age children receiving highly emetogenic chemotherapy.
Anticipatory nausea (AN) is reported by approximately 20% of patients at any one chemotherapy cycle and by 18-57% of patients by the fourth chemotherapy cycle.
Furthermore, commonly prescribed anti-emetic drugs are associated with significant adverse effects, such as sedation, extra-pyramidal side effects and hypotension (associated with dopamine antagonists), as well as headache, diarrhea or constipation (associated with 5-HT3 receptor antagonists). These medications have been shown to reduce vomiting episodes but they are less effective with nausea. A desirable attribute in any new anti-emetic medications would be the absence of significant adverse side effects, including nausea.
Adjuvant Treatments for CINV
Due to CINV side effects that persist despite standard usage of anti-emetic drugs, new adjuvant therapies are emerging and entering the market to address the issue of nausea. The first adjuvant on the market is Aprepitant (Emend®, Merck), which is a NK-1 receptor antagonist. The most common side effects of Emend® are weakness or fatigue, hiccups, diarrhea, nausea and constipation. Less common side effects are indigestion or heartburn, dizziness, dehydration, low blood pressure, abdominal pain, slow heart rate, throat pain and hot flashes. Emend® also has reported potential for adverse drug interactions with other prescription medications. Although clinical trials report increased efficacy, 50% of patients on the investigational arm for Aprepitant still had some degree of nausea and/or vomiting. The unmet medical need of CINV is acute in the adult and pediatric populations and is the reason why up to 50% of patients do not complete subsequent chemotherapy treatment.
Zindol DS – A Natural Adjuvant for CINV
Many patients reputedly use ginger for prevention or treatment of nausea and vomiting caused by chemotherapy for cancer (Quimby, 2007). Currently, a number of clinical trials have generated positive results that suggest that a regimen of ginger can significantly reduce the amount of nausea and vomiting during a course of chemotherapy (Pace, 1986; Sontakke, 2003; Levine et al, 2008; Ryan et al, 2009).
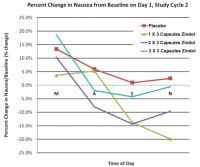
We recently completed a successful Phase 2/3 clinical trial on Zindol® as an adjuvant to conventional 5-HT3 anti-emetics (Ryan et al., 2009, 2011). The randomized, placebo-controlled, double-blind clinical trial assessed the efficacy of Zindol® in treating chemotherapy-related nausea in 576 cancer patients. All doses of Zindol® significantly reduced acute nausea severity compared to the placebo (p=0.003). No significant adverse events were reported; mild side effects such as heartburn, bruising/flushing, and rash were reported in less than 2% of patients.
Zindol® has several advantages over Emend® – a significant reduction in nausea with no significant side effects reported, simpler dosing regimen, no reported drug interactions, and lower cost.
References
- Levine M, Gillis M, Koch S, Voss A, Stern R and Koch K. (2008). Protein and ginger for the treatment of chemotherapy-induced delayed nausea. Journal of Alternative Complementary Medicine. 14(5): p. 545-51.
- Pace J. (1986). Oral ingestion of encapsulated ginger and reported self-care actions for the relief of chemotherapy-associated nausea and vomiting, in Dissertation Abstracts International University of Alabama.
- Quimby E. (2007). The use of herbal therapies in pediatric oncology patients: treating symptoms of cancer and side effects of standard therapies. J Pediatr Oncol Nurs. 24(1): 35-40.
- Ryan J, Heckler C, Roscoe J, Dakhil S, Kirshner J, Flynn P, Hickok J and Morrow G. (2011). Ginger (Zingiber officinale) reduces acute chemotherapy-related nausea: a URCC CCOP study of 576 patients. Supportive Care Cancer, August 2011.
- Ryan J, Heckler C, Dakhil S, Kirshner J, Flynn P, Hickok J and Morrow G. (2009). Ginger for chemotherapy-related nausea in cancer patients: A URCC CCOP randomized, double-blind, placebo-controlled clinical trial of 644 cancer patients. J Clin Oncol. 27:15s (suppl; abstr 9511).
- Sontakke S, Thawani V and Naik M. (2003). Ginger as an antiemetic in nausea and vomiting induced by chemotherapy: A randomized cross-over, double-blind study. Indian Journal of Pharmacology. 35(1): p. 32-36.