An enhanced St. John’s Wort product, which is standardized on the basis of serotonin re-uptake inhibition, for emotional well-being, relaxed outlook & positive mood*
 Xantol™ DS is an enhanced St. John’s Wort product, which is standardized by hyperforin content [US Patent No. 6,291,241]. Additionally, Aphios utilized its patented SuperFluids™ fractionation [US Patent Nos. 6,569,640 and 5,854,064] and manufacturing [US Patent Nos. 5,750,709 and 5,440,055] technologies to develop this product.
Xantol™ DS is an enhanced St. John’s Wort product, which is standardized by hyperforin content [US Patent No. 6,291,241]. Additionally, Aphios utilized its patented SuperFluids™ fractionation [US Patent Nos. 6,569,640 and 5,854,064] and manufacturing [US Patent Nos. 5,750,709 and 5,440,055] technologies to develop this product.
Isolation of Bioactive Ingredient
St. John’s Wort (Hypericum perforatum, SJW) is an over-the-counter herbal remedy, which is often utilized for emotional well-being. Hypericum perforatum is a bushy perennial with yellow flowers that bloom around St. John the Baptist’s day (June 24th). An oil extract of the fresh flowers contains liposoluble napthodianthrones and phloroglucinols, such as hypericin and hyperforin.
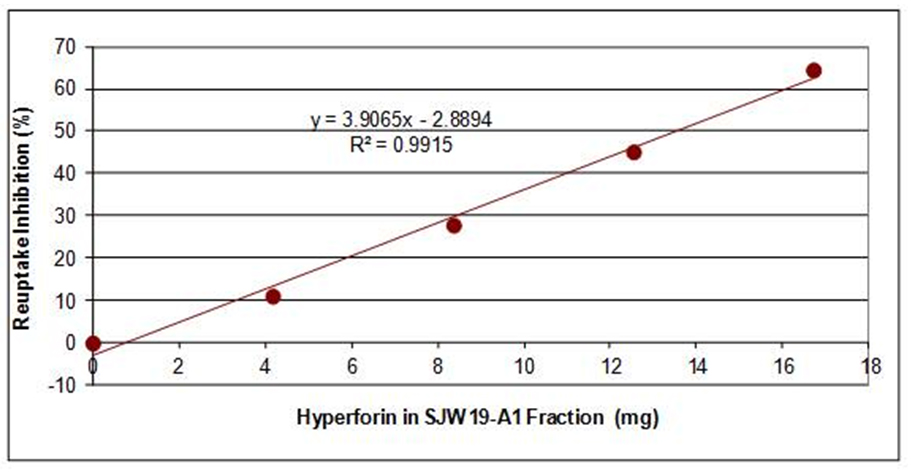
In our research, we identified and patented an enriched SJW fraction of Hypericum perforatum that is quite effective as a serotonin reuptake inhibitor (SRI). The bioactive ingredient was identified to be hyperforin through an in vitro serotonin reuptake inhibition assay and by HPLC chemical and mass spectral analyses.
In Vitro Serotonin Reuptake Inhibition Activity
Xantol™ DS has been shown in an in vitro serotonin reuptake inhibition assay to exhibit linear dose-response curves with respect to hyperforin content. Hyperforin is a clear, honey-colored and honey-like material that becomes waxy at 4°C; it is insoluble in water and only partially soluble in absolute ethanol.
Formulation, Permeability, Stability, and Bioavailability
The Xantol™ DS formulation contains natural antioxidants to improve stability, emulsifiers for improved bioavailability and is free of toxic organic solvents.
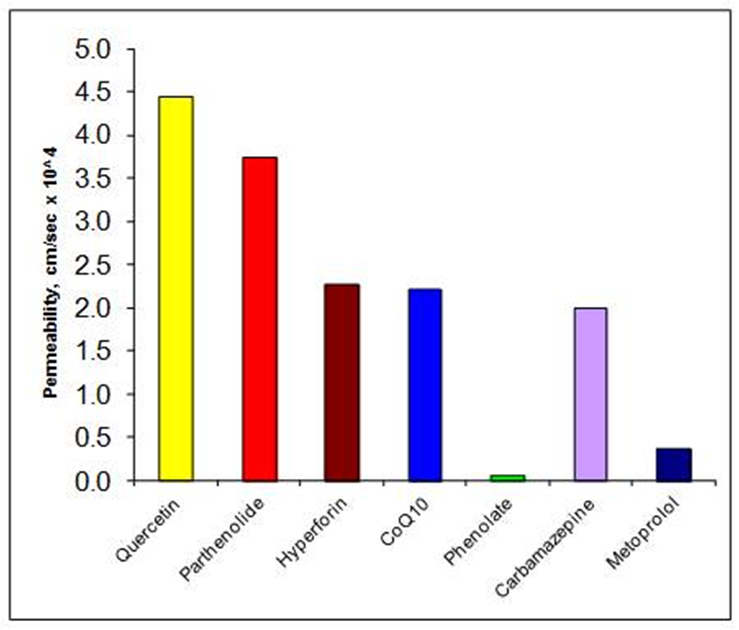
Permeability measurements, using the single-pass perfusion technique in rats, determined that Xantol™ DS is very permeable, perhaps 5 to 6 times higher permeability than metoprolol, which has been recommended as a “high permeability” internal standard.
Standardization and Manufacturing
Xantol™ DS is an enhanced, standardized formulation of St. John’s Wort based on hyperforin content and serotonin reuptake inhibition.
Xantol™ DS is manufactured from organically-grown Hypericum perforatum (St. John’s Wort) utilizing environmentally-friendly SuperFluids™ carbon dioxide technologies [US Patent Nos. 5,750,709 and 5,440,055]. This product is encapsulated in Licaps® liquid capsules by Capsugel®, Inc.
The capsule of this product is made from vegetables and does not contain any animal byproducts. The capsules are purged with nitrogen to further enhance stability. The capsules are contained in an opaque bottle to minimize light intrusion and improve shelf life. This product is free of toxic organic solvents and is an all-natural “green product.”
This product is manufacture following current Good Manufacturing Practices (GMP) of the U.S. Food and Drug Administration (FDA).
*This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent disease.
Xantol™ DS is a Trademark of Aphios Corporation.
Licaps® is a Registered Trademark of Capsugel, Inc.