A nanosomal formulation of paclitaxel, for Kaposi’s sarcoma and skin cancers
Dermos™ is a topical treatment for AIDS-associated Kaposi’s sarcoma (AIDS-KS) and other skin cancers as well as proliferative diseases of the skin utilizing a nanosomal formulation of a potent anticancer drug.
Current Therapies for Treating Kaposi’s Sarcoma and Their Side Effects
Liposomal formulations of daunorubicin and doxorubicin have been approved by the FDA for first-line and second-line systemic treatment of AIDS-KS. Liposomes are microscopic vesicles of phospholipid bilayers comprised of single or multiple lipid bilayers.
Paclitaxel, a unique anti-mitotic agent, has been approved by the FDA for systemic application in metastatic cases of breast cancer and refractory cases of ovarian cancer, and AIDS-KS.
These three drugs, while being very effective, are burdened by systemic toxicities such as neutropenia, myelosuppression, alopecia, nausea, and vomiting.
Topical Formulation for Treating Kaposi’s Sarcoma
A topical formulation of an antineoplastic drug that is effective against visual and subcutaneous Kaposi’s sarcoma will reduce the systemic use of these drugs, minimizing blood toxicity levels and improving a patient’s quality of life.
Nanosomes are ideal vehicles for the topical delivery of antineoplastic agents. Nanosomes™ are small, uniform liposomes that are less than one nanometer in diameter.
Dermos™ for the Treatment of AIDS-KS as Developed by:
- Comparing the deposition of paclitaxel into and across hairless mouse skin following in vitro and in vivo topical application of various formulations
- Validating the use of in vitro diffusion experiments to predict in vivo deposition behavior
- Comparing the kinetics of uptake into and across hairless mouse skin following topical in vivo application of various formulations
- Evaluating the effects of formulation composition on the deposition of these agents in hairless mouse skin
- In vivo evaluation of best topical preparation against KS Y-1 tumors induced in nude mice
Dermos™ – In Vitro Characterization
 To characterize the early steps accompanying apoptosis, KS cells were monitored by 3D epifluorescence confocal microscopy 48 hours after treatment with the topical preparation. Cells treated with PBS showed defined normal nucleoli. In contrast, KS Y-1 cells treated with the topical formulation exhibited overall induced reduction in cell size as well as diffuse staining and clumping of chromatin which is consistent with apoptosis.
To characterize the early steps accompanying apoptosis, KS cells were monitored by 3D epifluorescence confocal microscopy 48 hours after treatment with the topical preparation. Cells treated with PBS showed defined normal nucleoli. In contrast, KS Y-1 cells treated with the topical formulation exhibited overall induced reduction in cell size as well as diffuse staining and clumping of chromatin which is consistent with apoptosis.
Dermos™ – In Vivo Efficacy
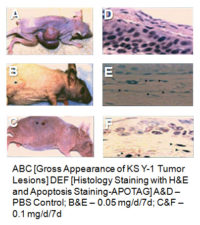 Immunodeficient mice [BNX-nude mice] were injected subcutaneously with neoplastic cell lines: KS Y-1 cells [5×106 cells] until the appearance of the tumor [7-10 days after post inoculation]. Nude mice were treated with PBS as controls, low and high doses of “topical” treatment: 0.05 – 0.1 mg/daily for 7 days. Topical treatment was given after tumor appearance with a slow release via osmotic pump [Alza, Alzet-osmotic pumps- 7days, 200 μL]. All the xenotransplanted mice were measured for size of the tumors, picture of the gross tumors and biopsies H&E staining and ApoTag for Apoptosis review.
Immunodeficient mice [BNX-nude mice] were injected subcutaneously with neoplastic cell lines: KS Y-1 cells [5×106 cells] until the appearance of the tumor [7-10 days after post inoculation]. Nude mice were treated with PBS as controls, low and high doses of “topical” treatment: 0.05 – 0.1 mg/daily for 7 days. Topical treatment was given after tumor appearance with a slow release via osmotic pump [Alza, Alzet-osmotic pumps- 7days, 200 μL]. All the xenotransplanted mice were measured for size of the tumors, picture of the gross tumors and biopsies H&E staining and ApoTag for Apoptosis review.
The topical treatment of Dermos™ has proven to be quite effective in vivo against xenotransplanted KS Y-1 cells in nude mice and was shown to cause apoptosis in vitro in KS Y-1 cells by 3D epifluorescence confocal microscopy.
Dermos™ Applications
Current therapies for Kaposi’s sarcoma, while being very effective, are burdened by systemic toxicities such as neutropenia, myelosuppression, alopecia, nausea, and vomiting. A topical formulation that is effective against visual and subcutaneous Kaposi’s sarcoma would reduce the systemic use of these drugs, minimizing blood toxicity levels and improving a patient’s quality of life.
The Dermos™ formulation with other active ingredients will find utility in melanoma, other types of skin cancers and proliferative diseases of the skin such as alopecia.