A non-toxic Vitamin D3 analog for hormone-refractory prostate cancer (HRPCA), designed to have increased antiproliferative effects while reducing systemic toxicity through increased specificity
Prostate Cancer Prevalence and Demographics
 Prostate cancer is the most prevalent cancer among men, and it is the second leading cause of cancer death among men in the United States. More than 500,000 cases are diagnosed annually. In the U.S. this year, 1 in 6 males will develop the disease and 30,000 others will die of it.
Prostate cancer is the most prevalent cancer among men, and it is the second leading cause of cancer death among men in the United States. More than 500,000 cases are diagnosed annually. In the U.S. this year, 1 in 6 males will develop the disease and 30,000 others will die of it.
Although prostate cancer mostly affects elderly men, the number of younger men with prostatic carcinoma is significant. In addition, the general increase in longevity has further emphasized the need for effective treatment of prostate cancer, especially for those forms that are androgen-insensitive and metastatic.
Current Prostate Cancer Treatments
Current clinical interventions for prostate cancer include surgical removal of the prostate and radiation therapy, with adverse side effects such as impotence, incontinence, and alopecia. On the other hand, the mainstay of chemotherapy includes androgen deprivation. However, no therapy is currently available for localized and metastatic prostate cancer that fails to respond to androgen therapy (in 9 to 30 months).
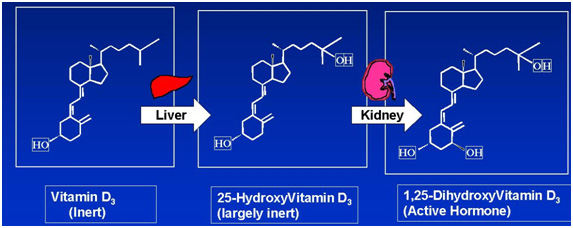
Vitamin D3 Epidemiology and Mechanism of Action
Epidemiological studies have shown that there is a correlation between lines of latitude and the incidence of prostate cancer – there is an increased incidence of prostate cancer with increased distance from the equator and reduced exposure to the sun, the UV rays of which stimulate the conversion of certain phospholipids into an inert Vitamin D3 in the skin. This problem is exacerbated for people of color, whose skin pigmentation absorbs UV radiation and reduces the generation of Vitamin D3.
The inert Vitamin D3 is then transported to the liver where a hydroxyl group [OH] is added to form a semi-hormone, and then to the kidney where an additional hydroxyl group [OH] is added to form the fully active Vitamin D3 hormone.
Numerous studies have shown that prostate cancer cells respond to the active hormone of Vitamin D3 formed in the kidney by enhancing differentiation and decreasing proliferation. However, the use of the active hormone is limited by the risk of toxicity (hypercalcemia and hypercalciuria) with significant loss of body weight.
Thus, less toxic Vitamin D3 analogs with potent antiproliferative activities are needed for prostate cancer and other cancers, as well as for autoimmune diseases such as multiple sclerosis and osteoporosis, which are linked to the nuclear Vitamin D Receptor (VDR).
APH-0701, a Nontoxic Vitamin D3 Analog
We are developing a nontoxic Vitamin D3 analog, APH-0701, which covalently links the bioactive hormone inside the ligand-binding pocket of the VDR. APH-0701 cross-links to the hormone-binding pocket of the VDR, thereby significantly reducing catabolic degradation. As a result, lower concentrations are needed to have an anti-tumor effect resulting in reduced toxicity.
APH-0701 has been shown by in vivo studies to be a strong anticancer agent in human prostate, kidney, pancreas, bladder, melanoma, and leukemia cancer cells, inducing apoptosis in these cells.
APH-0701 – In Vivo Efficacy and Toxicity
 In vivo studies have shown strong anticancer effects of APH-0701 against Hormone Refractory Prostate Cancer (HRPC) xenografts in nude mice at doses approximately 6.5 times less on a molar basis than the parent hormone, without significant toxicity (no significant weight loss, data not shown). APH-0701 thus demonstrates a strong translational potential as a therapeutic agent for HRPC and other cancers.
In vivo studies have shown strong anticancer effects of APH-0701 against Hormone Refractory Prostate Cancer (HRPC) xenografts in nude mice at doses approximately 6.5 times less on a molar basis than the parent hormone, without significant toxicity (no significant weight loss, data not shown). APH-0701 thus demonstrates a strong translational potential as a therapeutic agent for HRPC and other cancers.
APH-0701 Formulation and Development
In order to reduce acute and potential long-term side effects, Aphios® is encapsulating APH-0701 in phospholipid nanosomes using SuperFluids™ CFN technology in order to decrease toxicity, control release and improve therapeutic index.
We are developing APH-0701 for intravenous, nanotechnology delivery and an oil formulation (APH-1201) for the oral administration.