An orally bioavailable nanoformulation of betulinic acid that works as an HIV maturation inhibitor
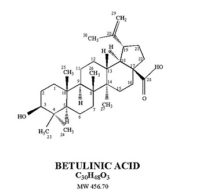 APH-0202 is an oral nanoformulation containing betulinic acid. A pentacyclic triterpene, betulinic acid is a component of the bark of the white birch tree, Betula alba. Phospholipid nanosomes can be utilized to enhance the formulation and efficacy of betulinic acid. Oral bioavailability can be enhanced by nanoencapsulating betulinic acid nanosomes and/or betulinic acid in biodegradable polymer nanospheres.
APH-0202 is an oral nanoformulation containing betulinic acid. A pentacyclic triterpene, betulinic acid is a component of the bark of the white birch tree, Betula alba. Phospholipid nanosomes can be utilized to enhance the formulation and efficacy of betulinic acid. Oral bioavailability can be enhanced by nanoencapsulating betulinic acid nanosomes and/or betulinic acid in biodegradable polymer nanospheres.
Betulinic Acid – A Unique HIV Maturation Inhibitor
 Betulinic acid has been shown to have potent anti-HIV activity with an EC50 of 1.4 µM and a therapeutic index of 9.3 by a novel mechanism of action. It prevents the entry of HIV into the target cell, but it may also act at the maturation stage.
Betulinic acid has been shown to have potent anti-HIV activity with an EC50 of 1.4 µM and a therapeutic index of 9.3 by a novel mechanism of action. It prevents the entry of HIV into the target cell, but it may also act at the maturation stage.
Betulinic acid and its derivatives have also demonstrated anti-infective bioactivity against herpesvirus, malaria, and leishmaniasis.
Formulation Difficulties Limiting Full Therapeutic Efficacy
Formulation problems have been encountered, as betulinic acid is insoluble in many solvents.
Derivatives of betulinic acid have been made and tested to circumvent this problem. Panacos Pharmaceuticals, Watertown, MA developed a water-soluble betulinic acid derivative [PA-457 or 3-O-(3′,3′-dimethylsuccinyl) betulinic acid] and tested it in Phase I and II clinical trials for HIV. PA-457 disrupts the late-stage viral maturation processes of HIV’s gag protein. The gag protein forms the capsid shell around a retrovirus’ RNA and establishes a viral core structure. Treatment with PA-457 causes this core structure to be defective and non-infectious.
Unfortunately, the clinical trials failed because of poor oral bioavailability associated with the PA-457 formulations.
Nanoformulation of Betulinic Acid for Improved Efficacy
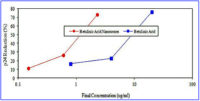 CFN technology was used to form betulinic acid nanosomes having a unimodal particle size distribution with a mean diameter of 180 nm ranging from 174 to 186 nm (95% confidence limits). A stock solution of the betulinic acid standard was prepared in dimethyl sulfoxide (DMSO). Serial 1:2 or 1:5 dilutions were tested in triplicate in an anti-HIV cytoprotection assay, beginning with 100 µg/mL. A sample of the empty nanosomes was also filtered through a 0.2 µm low protein binding filter and serial 1:2 dilutions were tested in triplicate.
CFN technology was used to form betulinic acid nanosomes having a unimodal particle size distribution with a mean diameter of 180 nm ranging from 174 to 186 nm (95% confidence limits). A stock solution of the betulinic acid standard was prepared in dimethyl sulfoxide (DMSO). Serial 1:2 or 1:5 dilutions were tested in triplicate in an anti-HIV cytoprotection assay, beginning with 100 µg/mL. A sample of the empty nanosomes was also filtered through a 0.2 µm low protein binding filter and serial 1:2 dilutions were tested in triplicate.
In an in vitro cytoprotection assay, betulinic acid inhibited p24 production by 76% at 20 µg/mL; the EC50was 6.72 µg/mL or 14.7 µM. The positive control 3TC resulted in 93% inhibition of p24 production at 0.2 µM, as expected.
The nanosomal formulation of betulinic acid inhibited p24 production by 73% at 2.3 µg/mL; the EC50 was 1.01 µg/mL or 2.2 µM (based on betulinic acid content). A second assay confirmed these results, showing that betulinic acid nanosomes (CANN-04) inhibit HIV at lower concentrations than betulinic acid alone and that the empty nanosomes (CANN-05) have a minimal effect on HIV.
Manufacturing of API, Betulinic Acid and Nanoformulation, APH-0202
Aphios Corporation has also developed an improved process for manufacturing betulin and betulinic acid from birch bark. The process utilizes CXP manufacturing technology and proprietary downstream purification processes. The research leading to these developments was made under Phase I & II Fast Track Small Business Innovative Research (SBIR) contracts from the National Cancer Institute (NCI), United States National Institutes of Health (NIH).
APH-0202, an orally bioavailable nanoformulation of betulinic acid that works as an HIV maturation inhibitor, can be manufactured using CFN technology and PNS technology, as described for APH-0201.