An alpha (α)-secretase modulator for ameliorating Alzheimer’s Disease pathophysiology and cognitive impairment with neuroprotection
Alzheimer’s Disease
 The symptoms of Alzheimer’s Disease (AD) are associated with progressive brain tissue shrinkage and death. In the early stages of AD, degeneration of cells in the hippocampus leads to short-term memory loss and reduced ability to perform routine tasks (cognitive disorders). As AD spreads through the cerebral cortex, judgment declines, emotional outbursts often occur and language skills become impaired.
The symptoms of Alzheimer’s Disease (AD) are associated with progressive brain tissue shrinkage and death. In the early stages of AD, degeneration of cells in the hippocampus leads to short-term memory loss and reduced ability to perform routine tasks (cognitive disorders). As AD spreads through the cerebral cortex, judgment declines, emotional outbursts often occur and language skills become impaired.
AD progression leads to the death of additional nerve cells and loss of synapses and gradually worsening behavior changes, such as wandering and agitation. In the final stages, patients lose the ability to recognize faces, communicate and control bladder and bowel movements. The average time from diagnosis to death is 4 to 8 years, although it may take 20 years or more for the disease to run its course.
While there is some understanding of this disorder, there is still a lack of preventive and therapeutic remedies. AD is the third major cause of death in America and among the highest in the industrial world. AD is a widespread and significant neurological disorder that affects more than 4.5 million Americans and more than 10 million people worldwide. Epidemiologically, AD is anticipated to increase with the demographics of the aging populations in the United States, Europe, and Japan. Experts estimate that 22 million people around the world and more than 8 million Americans will be afflicted with AD by 2025.
Current Alzheimer’s Disease Drugs
Five prescription drugs are currently approved by the US Food and Drug Administration (FDA) to treat people who have been diagnosed with Alzheimer’s. While these drugs may treat the symptoms of AD, none of these medications is a cure for the disease.
Four of these medications, including Aricept®, are called cholinesterase inhibitors and are prescribed for the treatment of mild to moderate AD. They may help delay or prevent symptoms from becoming worse for a limited time and may help control some behavioral symptoms. The fifth approved medication, known as Namenda®, is believed to work by regulating glutamate, another important brain chemical that, when produced in excessive amounts, can cause excitotoxicity that leads to brain cell death. All of the drugs on the market today have serious side effects.
Current Approaches Being Evaluated for Treating Alzheimer’s Disease Drugs
Current research in AD prophylaxis and therapy is centered on identification of methods to decrease Beta-amyloid (Aβ) and the assembly of tau tangles, biochemical ‘signatures’ of AD which are the basis of cognitive decline. Beta (β)-secretase inhibitors have been extensively investigated, but have been difficult to translate into effective clinical treatments for AD. A recent Phase II AD clinical trial with the β-secretase inhibitor, LY2886721, was even halted because of liver toxicity. Gamma (γ)-secretase inhibitors may conceivably also reduce Aβ accumulation. However, a recent Phase III clinical trial with the γ-secretase inhibitor, ‘Semagacestat’ (LY-450139), was also halted because of evidence for accelerated AD dementia. Most of the recent clinical AD trial failures have been with β- and γ-secretase inhibitors.
Alpha (α)-Secretase Approach for Treating AD
Alpha (α)-secretase is another enzyme in the neuronal pathway that positively influences amyloid precursor protein, (‘APP’) processing. Both β-secretase and γ-secretase cleave APP to form insoluble amyloid plaques (Aβ) that set in motion tau fiber assembly. In contrast, α-secretase cleaves APP into the harmless and more soluble product, ‘sAPP-α’, that actually supports new synapse formation and is more readily cleared from the brain. Thus, unlike current strategies which aim to suppress Aβ plaque formation by minimizing β- and γ-secretase activities, our strategy to activate α-secretase effectively eliminates the substrate for β- amyloid generation, and at the same time leads to positive amyloid precursor processing to both prevent and reduce Aβ plaques in AD.
APH-1104, a Novel α-Secretase Modulator and Potential AD Therapeutic
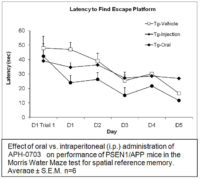
Aphios is developing APH-1104, a more potent analog of Bryostatin-1, which is neuroprotective by α-secretase activation via novel PKC isoforms, down-regulation of pro-inflammatory and angiogenic processes and the substitution of β-amyloid for its soluble and harmless relative, s-APPα. Aphios has successfully developed and patented efficient methods for manufacturing and formulating APH-1104. In 2008, Aphios investigated APH-1104, a less potent analog of APH-1104, as an intravenous formulation for Alzheimer’s disease in an open-label n=1 clinical trial in the Bahamas. Although limited, the preliminary results of this exploratory study were striking and encouraging.
In 2010, Aphios Corporation received a highly competitive 3-year, $2.85 million Fast Track Small Business Innovative Research (SBIR) grant from the National Institute on Aging (NIA), National Institutes of Health (NIH) to develop an oral formulation of APH-1104 for mild to moderate Alzheimer’s disease (AD) and cognitive disorders (CD). Aphios and its collaborators at Louisiana State University Health Sciences Center (LSUHSC-S) have now demonstrated by both in vitro mechanistic studies and in vivo efficacy studies that APH-1104 has tremendous clinical translation potential based on clinical safety and efficacy data. This research has discovered and identified even more potent analogs of APH-1104. We plan to develop a more potent analog, APH-1104 (Patent Pending), of APH-1104.
APH-1104 Product Development Plans:
We will develop APH-1104 as a therapeutic for mild to moderate Alzheimer’s disease (AD) and cognitive disorders (CD). We plan to utilize other analogs of APH-1104 as backups for APH-1104. Our milestones are as follows: (1) establish cGMP for the API and FDP at the pilot-scale level; (2) establish a drug master file, design IND enabling preclinical studies and Phase I/IIa clinical trials, and draft IND package; (3) conduct FDA-necessary IND-enabling preclinical in vivo studies, including toxicology, efficacy and pharmacology, under GLP; (4) perform stability testing of API and FDP under GLP; (5) file IND for conducting Phase I/IIa clinical trial of APH-1104; and (6) conduct Phase I and IIa clinical trials of oral APH-1104.
Other CNS Disorders:
APH-1104 and its analogs will also be evaluated and developed for other CNS disorders Hutchinson Disease, Parkinson’s disease, Down’s syndrome, and Glaucoma.