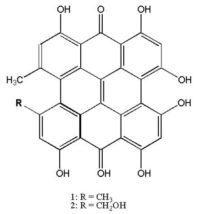
A photodynamic anti-viral and anti-HIV compound isolated from St. John’s Wort (Hypericum perforatum)
- Catalog No: APH-20013
- CAS Number: 548-04-9
- Chemical Formula: C30H16O8
- Molecular Weight: 504.45
- Purity: > 90 % determined by CP HPLC
- Appearance: Blue-black solid
- Solubility: Soluble in DMSO
- Stability: Stable under recommended storage conditions.
- Storage: -20ºC in the absence of light and oxygen
- Shipping: On ice (5ºC)
- Handling: Avoid strong oxidizing agents.
Source:
Hypericin is a naphthodianthrone, a red-colored anthraquinone-derivative, which, together with hyperforin, is one of the principal active constituents of St. John’s Wort (Hypericum perforatum). Hypericin is a photosensitive pigment found in St. John’s Wort.Hypericin is extracted from the flowers and buds of St. John’s Wort (H. perforatum) utilizing patented SuperFluids™ CXP technology [Castor, US Patent]. Then, normal phase chromatography is used to separate oils and other components from hypericin.
Biological Activity:
In St. John’s wort (H. perforatum), hypericin is a pigment that aids chlorophyll during photosynthesis. Hypericin has also been found in some hemipteran insects (Banks 1976), some protists, and buckwheat (Fagopyrum esculentum) (Duráan 2008).
Hypericin has shown promise as an anti-HIV and anticancer drug. Hypericin is a potential compound for use in photodynamic therapy, an innovative cancer treatment regime gaining popularity in the medical community. The National Cancer Institute describes photodynamic therapy as “a treatment that uses a drug, called a photosensitizer or photosensitizing agent, and a particular type of light. When photosensitizers are exposed to a specific wavelength of light, they produce a form of oxygen that kills nearby cells” (National Cancer Institute). Hypericin as a photosensitizer has been shown to induce apoptosis and necrosis in cancer cells using photodynamic therapy (Agostinis 2002).
In laboratory studies, hypericin has demonstrated anti-retroviral activity using a method of action that does not rely on inhibition, inactivation, or modification of reverse transcriptase. The findings of the one study in particular “suggest that, unlike nucleoside analogues, [hypericin and pseudohypericin] directly inactivate the virions or interfere with assembly or shedding of assembled viral particles” (Meruelo, 1998). Hypericin is not limited to just HIV. Studies suggest that it is equally effective at inactivating enveloped viruses such as herpes simplex and influenza A (Tang 1990).
References:
Agostinis P, Vantieghem A, Merlevede W and de Witte P. (2002) Hypericin in Cancer Treatment: More Light on the Way. The International Journal of Biochemistry and Cell Biology. 34(3), 221-241.
Banks H, Cameron D and Raverty W. (1976) Chemistry of the Coccoidea. II. Condensed polycyclic pigments from two Australian pseudococcids (Hemiptera). Australian Journal of Chemistry. 29(7) 1509-1521.
Duráan N and Song P. (2008) Hypericin and its photodynamic action. Phytochemistry and phytobiology. 43(6), 677-680.
Meruelo D, Lavie G and Lavie D. (1988) Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: Aromatic polycyclic diones hypericin and pseudohypericin. Proceedings of the National Academy of Sciences. 85, 5230-5234.
National Cancer Institute. (2012) Cancer Drug Information: Paclitaxel. www.cancer.gov.
Tang J, Colacino J, Larsen S and Spitzer W. (1990) Virucidal Activity of Hypericin Against Enveloped and Non-enveloped DNA and RNA Viruses. Antiviral Research. 13(6), 313-325.