An alkaloid isolated from the corn lily (Veratrum californicum) is a Sonic Hedgehog gene pathway antagonist with potential anticancer properties
- Catalog No: APH-05011
- CAS Number: 4449-51-8
- Chemical Formula: C27H41NO2
- Molecular Weight: 411.63
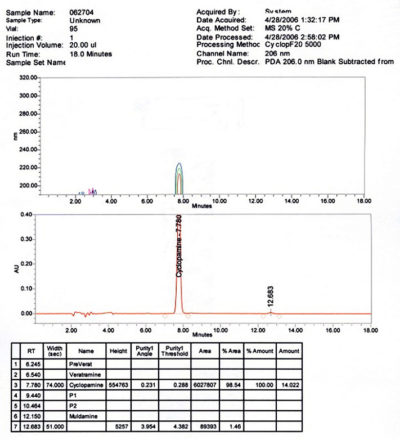
- Purity: > 99% determined by HPLC
- Appearance: White to off-white crystals
- Solubility: DMSO ≥ 6 mg/mL; Water < 1 mg/mL; Ethanol ≥ 39 mg/mL
- Stability: Stable over extended period at -20°C.
- Storage: -20°C
- Shipping: On ice (5°C)
- Handling: Avoid exposure to skin and eyes.
Source:
Cyclopamine (11-deoxojervine) is a steroidal alkaloid isolated from the corn lily (Veratrum californicum) that grows wild in the mountains and deserts of Nevada and California.
Cyclopamine was named for the one-eyed lambs were born to sheep that grazed on wild corn lily at a farm in Idaho.
In 1957, the US Department of Agriculture started a multi-year investigation that led to the identification of cyclopamine as the cause of the birth defect (Herper, 2005). Investigators moved the sheep herds around to determine if the development of a single eye (cyclopia) was genetic.
Eventually, it was discovered that the mutation was caused by sheep chewing on the roots of the corn lily shrub. The responsible chemical was isolated and determined to be an amine. Having been the determinant cause of cyclopia and being an amine, the compound was named cyclopamine.
Interestingly, the landscape in certain parts of Greece and the Mediterranean is quite similar to the mountains and deserts of Nevada and California. There is a high likelihood that plants similar to corn lily were present in these areas during ancient times, leading to one-eyed animals and the genesis of one of the lasting image of the Cyclops in Homer’s Odyssey and Greek mythology.
Aphios isolates and manufactures cyclopamine utilizing near-critical and supercritical fluids using CXF and CXP enabling technology platforms followed by proprietary segmentation chromatography.
Biological Activity:
Cyclopamine is a teratogen that causes fatal birth defects. It can prevent the fetal brain from dividing into two lobes (holoprosencephaly) and cause the development of a single eye (cyclopia). Cyclopamine acts as a primary inhibitor of the so-called “hedgehog” signal-transduction pathway in cells. Cyclopamine inhibits the hedgehog signaling pathway (Hh) by influencing the balance between the active and inactive forms of the smoothened protein.
This pathway, named for the ligand for the signal protein, is used by cells to help them react to external chemical signals. The pathway carries out important functions in embryonic development and when it goes awry, deformities can occur.
Cyclopamine is useful in studying the role of Hh in normal development, and as a potential treatment for certain cancers in which Hh is over-expressed.
Errant activation of the Hh pathway can also trigger cancer in adult humans, leading to basal cell carcinoma, medulloblastoma, rhabdomyosarcoma, and prostate, pancreatic and breast cancers. A way of controlling the pathway using cyclopamine could turn this problem on its head and provide a way to treat cancer. Many anticancer drugs are paradoxically carcinogenic in healthy individuals (Bradley, 2009).
Cyclopamine is currently being investigated as a treatment agent in basal cell carcinoma, medulloblastoma and rhabdomyosarcoma, tumors that result from excessive Hh activity (Taipale et al., 2000) and glioblastoma, and also as a treatment agent for multiple myeloma.
The cyclopamine derivative IPI-926 (also known as saridegib) is being evaluated in clinical trials by Infinity Pharmaceuticals for the treatment of various types of cancer (Pipeline: IPI-926). Infinity was testing IPI-926 with gemcitabine, in comparison to placebo with gemcitabine. Infinity Pharmaceuticals Inc. has voluntarily halted a Phase 2 study of its drug target to treat metastatic pancreatic cancer, after early results indicated the goal endpoint of overall survival would not be met. The IPI-926 combination showed a higher disease rate than the placebo combination, with Infinity’s combination indicating a median survival rate less than the six-month median of gemcitabine alone.
In January, 2012, the FDA approved Genentech’s Erivedge™ (vismodegib) that works by inhibiting the hedgehog pathway as an oral, once-daily medication for adults with locally advanced and metastatic advanced basal cell carcinoma. Erivedge™ will carry a boxed warning about a potential risk of death or severe birth effects to a fetus. The most common side effects are muscle spasms, hair loss, weight loss, nausea, diarrhea, fatigue, distorted sense of taste or loss or taste, decreased appetite, constipation, vomiting and aching joints.
References:
Bradley D. (2009). Keeping an eye on anticancer drug. NMR Knowledge Base.
Herper M. (2005). The Curious Case of The One-Eyed Sheep Forbes.com.
Pipeline: IPI-926. Infinity Pharmaceuticals.
Taipale J; Chen J, Cooper M, Wang B, Mann R, Milenkovic L, Scott M and Beachy P. (2000). Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature PubMed. 406 (6799): 1005–9. Doi:10.1038/35023008. PMID 10984056.