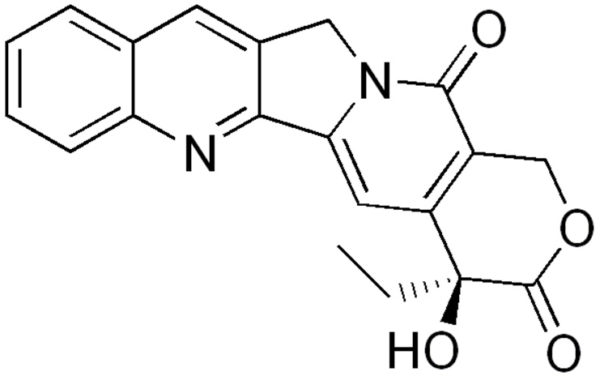
A potent topoisomerase I inhibitor and anticancer compound isolated from the Chinese Happy Tree (Camptotheca acuminata)
- Catalog No: APH-08041
- CAS Number: 7689-03-4
- Chemical Formula: C20H16N2O4
- Molecular Weight: 348.36
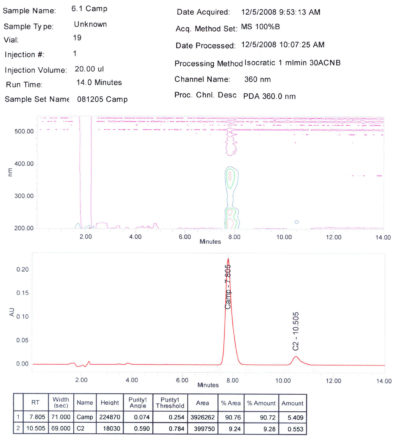
- Purity: > 90 % determined by CP HPLC
- Appearance: White powder
- Solubility: Soluble in ethanol, methanol, acetonitrile, DMSO and methylene chloride
- Stability: Stable at recommended storage conditions.
- Storage: 2-8ºC
- Shipping: Ambient
- Handling: Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Where dust is formed, provide exhaust ventilation.
Source:
Camptothecin is a natural alkaloid found in the bark, stem and leaves of the Chinese happy tree, Camptotheca acuminata, which is native to China. C. acuminata also goes by the names cancer tree and tree of life. These names are appropriate considering the anticancer properties of camptothecin. The Chinese have used C. acuminata in traditional medicine for thousands of years. It was commonly prescribed for psoriasis, leukemia and liver, gallbladder, spleen and stomach diseases (Chen, 2007).At Aphios, Camptothecin is extracted from the bark, stem and leaves of C. acuminata utilizing patented SuperFluids™CXP technology [Castor, US Patent] followed by segmentation chromatography.
Biological Activity:
Camptothecin (CPT) is a potent topoisomerase I inhibitor and an anticancer agent that was originally isolated from the bark and stem wood of C. acuminata, a small tree native to China. This compound displays unprecedented antitumor activities against leukemia, human colon cancer and a variety of solid tumor systems. In particular, camptothecin was discovered to trap the cleavable complex between topoisomerase I and DNA (Hertzberg et al., 1989). Camptothecin is a unique topoisomerase I inhibitor which, while very effective, is not yet completely understood. In addition, camptothecin inhibits the replication of HIV and other viruses.
 Camptothecin binds to and inhibits the action of mammalian DNA topoisomerase I (Hsiang et al., 1985). Topoisomerase I is an enzyme that is critical to DNA replication. During replication, topoisomerase I unwinds and separates the two strands of DNA to form two single strands. Camptothecin binds to Asp533 and Arg364 in topoisomerase I, as well as cytosine from the + DNA strand, forming a topoisomerase cleavage complex (Pommier et al., 2003). This complex arrests DNA replication in the S phase of cell growth and triggers apoptosis (Hsiang et al., 1989).
Camptothecin binds to and inhibits the action of mammalian DNA topoisomerase I (Hsiang et al., 1985). Topoisomerase I is an enzyme that is critical to DNA replication. During replication, topoisomerase I unwinds and separates the two strands of DNA to form two single strands. Camptothecin binds to Asp533 and Arg364 in topoisomerase I, as well as cytosine from the + DNA strand, forming a topoisomerase cleavage complex (Pommier et al., 2003). This complex arrests DNA replication in the S phase of cell growth and triggers apoptosis (Hsiang et al., 1989).
Depending on the pH of the aqueous solution, CPT can exist in two forms, the closed and open lactone forms. Only the closed lactone form (stable in acid) has anticancer properties. At higher pH’s (>5.5) the closed form can be hydrolyzed to the biologically inactive open lactone form (Ziomkowska et al., 2006)
The inactive open lactone form binds strongly and irreversibly to human serum albumin (HAS) present in blood (pH 7.4) and the amount of active lactone present in blood after 2 hrs is ca. 5% (Kruszewski et al., 2002). The rates of hydrolysis of CPT in a PBS (pH 7.4) and a HSA (pH 7.4) solution at 37ºC were measured. It was determined that there was essentially no closed lactone form present in the HSA solution after 2 hrs and less than 10% remaining in the PBS solution (Ziomkowska et al., 2006). These results indicate that the open lactone form is binding strongly with the HSA and is being pulled out of solution quickly affecting the equilibrium and allowing the hydrolysis to go to completion.
Camptothecin full therapeutic activity has been limited by poor water solubility and the aqueous instability of the lactone ring moiety. Ring opening will cause loss of biological activity of this compound. Preferential binding of the biologically inactive carboxylate form with human serum albumin further hinders drug effectiveness (Burke and Mi, 1994). With a closed ring lactone form, camptothecin is very water insoluble. Because of this water insolubility, it was formulated as ring-opened sodium salt in early clinical trials. Administration of the soluble sodium salt of camptothecin proved to be ineffective and delayed the development of this potent anticancer compound.
In an acidic environment, CPT was activated as a result of ring closure, which caused high rates of severe, unpredictable hemorrhagic cystitis and gastritis and direct mucosal toxicity in acidic urothelial and gastric tissues. These toxicities, probably driven by high CPT concentrations, in addition to poor efficacy led to suspension of CPT’s development for several decades. As a result of this, camptothecin analogs that exhibit a superior solubility profile while retaining antitumor activity were developed. These goals are frequently at odds, as increased hydrophilicity engenders greater exposure to the deactivating aqueous environment (Burke et al., 1993). Several promising clinical candidates, such as topotecan (Hycamtin), CPT-11 (Irinotecan, Camptosar), and 9-aminocamptothecin, have emerged (Potmesil, 1994); topotecan and CPT-11 have been approved by the FDA for colorectal, cervical, ovarian and other cancers.
Water soluble camptothecin analogs have yet to prove as effective as the native compound and some of its hydrophobic analogs. For example, researchers at Glaxo (Emerson, et al., 1995) have reported on the in vivo activity of two new water soluble camptothecin analogs. Even at the maximum active dose, no cures were obtained. This may be due to the instability of the lactone pharmaphore in aqueous media in general and in plasma in particular, the latter due at least in part to binding with human serum albumin.
Liposome encapsulated camptothecin can serve as a useful drug delivery system for solubilizing camptothecin, while retaining both its lactone ring and antitumor activity. In studies with CPT and liposomal bilayers, it was observed that the open lactone form of CPT was stable when associated within a liposomal bilayer for 48 hr in a PBS solution. This indicates that the lactone ring is embedded within the bilayer making it un-accessible to the hydrolytic aqueous phase (Burke, 1992).
References:
Burke T, Staubus A, Mishra A and Malak H. (1992) Liposomal stabilization of camptothecin’s lactone ring. J. Am. Chem. Soc. 114: 8318-8319.
Burke T, Mishra A, Wani M and Wall M. (1993) Lipid Bilayer Partitioning and Stability of Camptothecin Drugs. Biochemistry. 32, 5352-64.
Burke T and Mi Z. (1994) The Structural Basis of Camptothecin Interactions with Human Serum Albumin: Impact on Drug Stability. J. Medicinal Chemistry. 37, 40-6.
Chen K. (2007). Baran Group Meeting. Scripps Research Institute.
Emerson D, Besterman J, Brown H, Evans M, Leitner P, Luzzio M, Shaffer J, Sternbach D, Uehling D and Vuong A. (1995) In vivo Antitumor Activity of Two New Seven-Substituted Water-Soluble Camptothecin Analogues. Cancer Research. 55, 603-9.
Hertzberg R, Caranfa M and Hecht S. (1989) On the Mechanism of Topoisomerase I Inhibition by Camptothecin: Evidence for binding to an enzyme-DNA Complex. Biochemistry. 28, 4629-38.
Hsiang Y, Hertzberg R, Hecht S and Liu L. (1985) Camptothecin Induces Protein-linked DNA Breaks via Mammalian DNA Topoisomerase I. The Journal of Biological Chemistry. 260, 14873-14878.
Hsiang Y, Lihou M and Liu L. (1989) Arrest of Replication Forks by Drug-stabilized Topoisomerase I-DNA Cleavable Complexes as a Mechanism of Cell Killing by Camptothecin1. Cancer Research. 49, 5077-5082.
Kruszewski S and Burke T. (2002) Camptothecins Affinity to HAS and Membranes Determined by Fluorescence Antisotropy Measurements.
Pommier Y, Redon C, Rao V, Seiler J, Sordet O, Takemura H, Antony S, Meng L, Liao Z, Kohlhagen G, Zhang H and Kohn K. (2003) Repair of and checkpoint response to topoisomerase I-mediated DNA damage. Mutation Research. 532, 173-203.
Potmesil M. (1994) Camptothecins: From Bench Research to Hospital Wards. Cancer Research. 54, 1431-9.
Shimada Y, Yoshino M, Wakui A, Nakao I, Futatsuki k, Sakata Y, Kambe M, Taguchi T and Ogawa N. (1993) Phase II study of CPT-11, a new camptothecin derivative, in metastatic colorectal cancer. CPT-11 Gastrointestinal Cancer Study Group. Journal of Clinical Oncology. 11, 909-913.
Wagener D, Verdonk H, Dirix L, Catimel G, Siegenthaler P, Buitenhuis, Mathieu-Boue A and Verweij J. (1995) Phase II trial of CPT-11 in patients with advanced pancreatic cancer, an EORTC early clinical trials group study. Annals of Oncology. 6, 129-132.
Ziomkowska B, Kruszewski S, Siuda R and Cyrankiewicz M. (2006) Deactivation Rate of Camptothecin Determined by Factor Analysis of Steady-State Fluorescence and Adsorption Spectra. Optica Applicata. 36: 137-146.