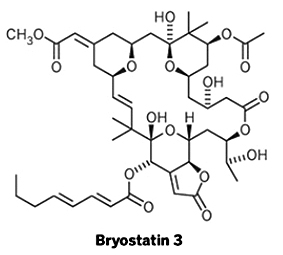
A Protein Kinase C modulator isolated from the bryozoan Bugula neritina
- Catalog No: APH-09063
- CAS Number: 143370-84-7
- Chemical Formula: C46H64O17
- Molecular Weight: 889.00
- Purity: > 95% determined by HPLC
- Appearance: White crystalline solid
- Solubility: Soluble in methanol and ethanol
- Stability: Stable as a solid over extended period at -20°C.
- Storage: -20°C
- Shipping: On ice (5°C)
- Handling: Avoid exposure to oxygen and direct sunlight.
Source:
Bryostatin 3 is one of a series of cyclic macrolides isolated from the marine bryozoan Bugula neritina (Order Cheilostomata). This arborescent bryozoan is found in temperate and subtropical environments worldwide, but only B. neritina from California and the Gulf of Mexico is known to contain Bryostatins 1, 2 and 3 that are characterized by the C- 20 (E,E)-octa-2-dienoate ester (Pettit, 1985). Since their discovery, bryostatins are postulated to be the product of a bacterial symbiont of the bryozoan (Davidson et al., 2001)
Bryostatin 3, a macrolide lactone, was first isolated and structurally characterized by Pettit et al. in 1983. Since then, its structure has been revised to correspond to 16 mass units less than Bryostatin 1 due to structural differences in the C-19/C-23 pyran ring (Schaufelberger et al., 1991a). Instead of a methoxy group, it contains a lactone ring system not observed in other bryostatins (Schaufelberger et al., 1991a).
Schaufelberger et al. (1991b) reported a novel scheme for the large-scale isolation of bryostatin from B. neritina. The reported procedure consists of several organic phase extraction and chromatographic purification steps that can be used for the isolation of Bryostatin 3.
Aphios (Castor, 1995, 1997, 1998, 2001) has improved the isolation of Bryostatin 3 from B. neritina by utilizing near-critical and supercritical fluids as an alternative to conventional organic solvents techniques, which are time consuming, labor intensive and environmentally insensitive even at a laboratory scale.
Biological Activity:
Bryostatin 3, unlike Bryostatin 1, has not been extensively studied for its bioactivity. It was originally isolated from B. neritina based on its antineoplastic activity (Pettit et al., 1983). It shows phorbol dibutyrate receptor displacement activity similar to that of Bryostatin 1 (Schaufelberger et al., 1991a).
The bryostatins are known to bind and activate Protein Kinase C (PKC), though they differ from other phorbol ester tumor promoters in that they act as agonists or antagonists of PKC activity (Mackanos et al., 1991). Bryostatin 3 was found to act as an antagonist to phorbol ester tumor promoter TPA, preventing the inhibition of GH4 cell proliferation, similar to what has been observed in Bryostatin 1 (Mackanos et al., 1991). Unlike Bryostatin 1, Bryostatin 3 also enhanced cell-substratum adhesion and did not block the increased adhesion observed from TPA (Mackanos et al., 1991).
References:
Castor T. (1995, 1997). Method and Apparatus for Extracting Taxol from Source Materials. US Patent No. 5,440,055 and European Patent No. 689,537.
Castor T. (1998). Method and Apparatus for Isolating Therapeutic Compositions from Source Materials. US Patent No. 5,750,709.
Castor T. (2001). Supercritical Fluid Isolation of Bryostatin 1. SBIR Phase II Final Report. SBIR Grant No. 5 R44 CA 64017-03.
Davidson S, Allen S, Lim G, Anderson C and Haygood M. (2001). Evidence for the biosynthesis of bryostatins by the bacterial symbiont “Candidatus Endobugula sertula” of the bryozoan Bugula neritina. Appl. Environ. Microbiol. 67, 4531-4537.
Mackanos E, Pettit G and Ramsdell J. (1991). Bryostatins selectively regulate protein kinase C- mediated effects on GH4 cell proliferation. J Biol Chem. 266: 11205-11212.
Pettit G, Herald C, and Kamano Y. (1983). Antineoplastic agents. 93. Structure of the Bugula neritina (marine bryozoan) antineoplastic component Bryostatin 3. J Org Chem 48: 5354-5356.
Schaufelberger D, Chmurny G, Beutler J, Koleck M, Alvarado B, Schaufelberger B, and Muschik G. (1991a). Revised structure of Bryostatin 3 and isolation of the Bryostatin 3 26-ketone from Bugula neritina. J Org Chem 56: 2895-2900.
Schaufelberger D, Koleck M, Beutler J, Vatakis A, Alvarado A, Andrews P, Marzo L, Muschik G, Roach J, Ross J, Lebherz W, Reeves M, Eberwein R, Rodgers L, Testerman R, Snader K and Forenza S. (1991b). The large scale isolation of Bryostatin 1 from Bugula neritina following good manufacturing practices. J. Nat. Prod. 54, 1265-1270.