A potent Protein Kinase C modulator isolated from the bryozoan Bugula neritina
- Catalog No: APH-09062
- CAS Number: 87745-28-6
- Chemical Formula: C45H66O16
- Molecular Weight: 863.01
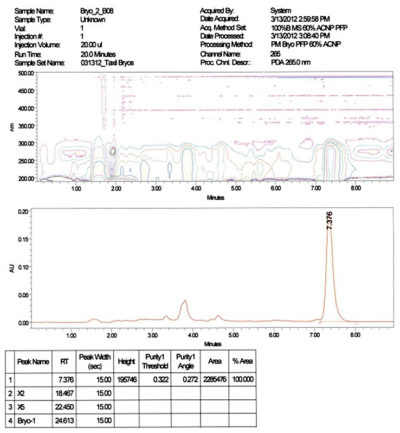
- Purity: > 95% determined by HPLC
- Appearance: White crystalline solid
- Solubility: Soluble in methanol and ethanol
- Stability: Stable as a solid over extended period at -20°C.
- Storage: -20°C
- Shipping: On ice (5°C)
- Handling: Avoid exposure to oxygen and direct sunlight.
Source:
Bryostatin 2 is one of a series of cyclic macrolides isolated from the marine bryozoan Bugula neritina (Order Cheilostomata). This arborescent bryozoan is found in temperate and subtropical environments worldwide, but only B. neritina from California and the Gulf of Mexico is known to contain Bryostatins 1, 2, 3, 13 and 15 that are characterized by the C- 20 (E,E)-octa-2-dienoate ester (Pettit et al., 1983)
Bryostatin 2, a macrolide lactone, was first isolated from the bryozoan B. neritina by Pettit et al. (1983) and has recently been postulated to be a product of a bacterial symbiont present in the bryozoan (Davidson et al., 2001).
Schaufelberger et al. (1991) reported a novel scheme for the large-scale isolation of Bryostatin 1 from the bryozoan B. neritina. The reported procedure consists of several organic phase extraction and chromatographic purification steps that can be utilized through slight modifications for the isolation of Bryostatin 2. Subsequently, Bryostatin 2 can be converted to Bryostatin 1 by a selective protection and deprotection strategy of the C-26 hydroxyl group (Pettit, 1991).
Aphios (Castor, 1995, 1997, 1998, 2001) has improved the isolation of Bryostatin 2 from B. neritina utilizing near-critical and supercritical fluids as an alternative to conventional organic solvents techniques, which are time consuming, labor intensive and environmentally insensitive even at a laboratory scale.
Biological Activity:
Bryostatin 2 is a macrocyclic lactone with anti-tumor properties and a potent activator of Protein Kinase C (PKC), which inhibits the growth of A549 cells (Dale et al., 1989). Bryostatin 2 has been documented to compete with phorbol ester binding to PKC at a Ki= 3.4 nM, a Ki value 1 order magnitude higher than Bryostatin 1, and antagonizes phorbol ester action (Jalava et al., 1990). Bryostatin 2 also exhibits growth inhibitory activity against several human cancer cell lines. It has been shown to inhibit DNA synthesis at 100 nM in SH-SY5Y human neuroblastoma cells (Jalava et al., 1990).
Other studies have shown that Bryostatin 2 can induce arachidonic acid release (Dell’Aquila et al., 1988). Bryostatin 2, along with Bryostatin 1, has been shown to synergistically act with recombinant B cell stimulatory factor-1 to cause differentiation and cytotoxic T lymphocyte development in naïve, resting lymph node T cells (Trenn et al., 1988).
References:
Castor T. (1995,1997). Method and Apparatus for Extracting Taxol from Source Materials. US Patent No. 5,440,055 and European Patent No. 689,537.
Castor T. (1998). Method and Apparatus for Isolating Therapeutic Compositions from Source Materials. US Patent No. 5,750,709.
Castor T. (2001). Supercritical Fluid Isolation of Bryostatin 1. SBIR Phase II Final Report. SBIR Grant No. 5 R44 CA 64017-03.
Dale I, Bradshaw T, Gescher A and Pettit G. (1989). Comparison of effects of Bryostatin 1 and 2 and 12-O-Tetradecanoylphorbol-13-acetate on Protein Kinase C activity in A549 human lung carcinoma cells. Cancer Res. 49: 3242-3245.
Davidson S, Allen S, Lim G, Anderson C and Haygood M. (2001). Evidence for the Biosynthesis of Bryostatins by the Bacterial Symbiont “Candidatus Endobugula sertula” of the Bryozoan Bugula neritina. Appl. Environ. Microbiol. 67, 4531-4537.
Dell’Aquila M, Herald C, Kamano Y, Pettit G and Blumberg P. (1988). Differential effects of bryostatins and phorbol esters in arachidonic acid metabolite release and epidermal growth factor binding in C3H 10T½ Cells. Cancer Res. 48: 3702-3708.
Jalava A, Heikkilä J, Akerlind G, Pettit G and Akerman K. (1990). Effects of bryostatins 1 and 2 on morphological and functional differentiation of SH-SY5Y human neuroblastoma cells. Cancer Res. 50: 3422-3428.
Pettit G. (1991). Synthetic conversion of Bryostatin 2 into Bryostatin 1. US Patent No. 5,072,004.
Pettit G, Herald C, Kamano Y, Gust D and Aoy-agi R. (1983). The structure of Bryostatin 2 from the marine bryozoan Bugula neritina. J. Am. Chem. Soc. 104, 6846–6848.
Schaufelberger D, Koleck M, Beutler J, Vatakis A, Alvarado A, Andrews P, Marzo L, Muschik G, Roach J, Ross J, Lebherz W, Reeves M, Eberwein R, Rodgers L, Testerman R, Snader K and Forenza S. (1991). The large scale isolation of Bryostatin 1 from Bugula neritina following good manufacturing practices. Journal of Natural Products. 54, 1265-1270.
Trenn G, Pettit G, Takayama H, Hu-Li J and Sitkovsky M. (1988). Immunomodulating properties of a novel series of protein kinase C activators. The bryostatins.