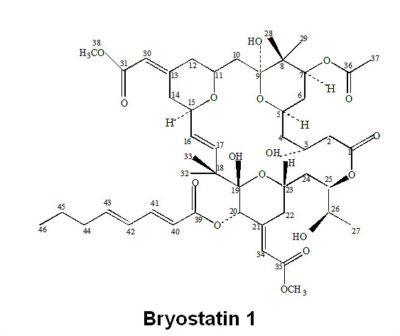
A potent Protein Kinase C modulator isolated from the bryozoan Bugula neritina
- Catalog No: APH-09061
- CAS Number: 83314-01-6
- Chemical Formula: C47H68O17
- Molecular Weight: 905.04
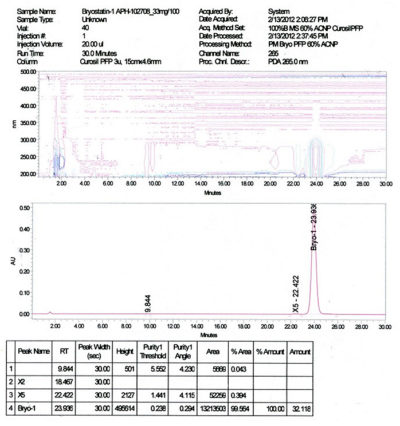
- Purity: > 99% determined by HPLC
- Appearance: White crystalline solid
- Solubility: Soluble in methanol and ethanol
- Stability: Stable as a solid over extended period at -20ºC.
- Storage: -20ºC
- Shipping: On ice (5ºC)
- Handling: Avoid exposure to oxygen and direct sunlight.
Source:
Bryostatin 1 is one of a series of cyclic macrolides isolated from the marine bryozoan Bugula neritina (Order Cheilostomata). This arborescent bryozoan is found in temperate and subtropical environments worldwide, but only B. neritina from California and the Gulf of Mexico is known to contain Bryostatins 1, 2 and 3 that are characterized by the C- 20 (E,E)-octa-2-dienoate ester (Pettit, 1985)
Bryostatin 1, a macrolide lactone, was first isolated from the bryozoan Bugula neritina by Pettit et al. (1985) and recently postulated to be produced by a bacterial symbiont of the bryozoan (Davidson et al., 2001).
Schaufelberger et al. (1991) reported a novel scheme for the large-scale isolation of bryostatin from the bryozoan Bugula neritina. The reported procedure consists of several organic phase extraction and chromatographic purification steps.
Aphios (Castor, 1995, 1997, 1998, 2001) has improved the isolation of Bryostatin 1 from Bugula neritinautilizing near-critical and supercritical fluids as an alternate to conventional organic solvents techniques which are time consuming, labor intensive and environmentally insensitive even at a laboratory scale.
Biological Activity:
Bryostatin 1 has undergone several Phase I and Phase II clinical trials against melanomas, lymphomas and renal cancers by the National Cancer Institute in the United States and by the Cancer Research Campaign in Great Britain (Newman, 1995; Philip et al., 1993; and Prendiville et al., 1993; Clamp et al., 2002; Zonder et al., 1999; Varterasian et al., 2000).
In vitro studies have shown that Bryostatin 1 is a potent antileukemic agent that works by a unique and unusual mechanism. This compound exhibits selective activity against leukemias and directly stimulates bone marrow progenitor cells to form colonies that functionally activate neutrophils (Suffness et al., 1989). This combined activity is unusual because most cytotoxic anticancer agents are toxic to bone marrow. The mechanism of activity is unknown but it may be related to the ability of the bryostatins to modulate the protein kinase C receptor.
Bryostatin 1 exhibits a high affinity for PKC and displaces phorbol esters from PKC at low nano- to picomolar levels (Hennings et al., 1987; Mutter et al., 2000). Many other biological effects have also been shown at the subnanomolar level. The established lack of tumor promotion and the fact that Bryostatin 1 is already in clinical development as a tumor suppressor has prompted exploration of its use as a modifier of APP metabolism and its capacity to enhance cognition, both effects that appear to be mediated by PKC activation (Etcheberrigaray et al., 2004; Olds et al., 1993; Favit et al., 1998; Alkon et al., 2005).
Bryostatin 1 activates HIV-1 gene replication through an NF-κB-dependent pathway in chronically infected macrophage cell lines (Qatsha et al., 1993; Vlach and Pitha 1992), and downregulates the expression of CD4 in T cell lines (Boto et al., 1991). Indeed, reactivation of HIV-1 latency in T and other lymphoid cells requires cell activation and it has been demonstrated that Bryostatin 1 activates resting humans’ T cells (Trenn et al., 1988) and enhances the maturation of human dendritic cells through a PKC-dependent pathway (Do et al., 2004).
Using Jurkat-LAT-GFP cells, a tractable model of HIV-1 latency, we have found that Bryostatin 1 reactivates HIV-1 through a classical PKC-dependent pathway. In addition, Bryostatin 1 downregulates the expression of the HIV-1 co-receptors CD4 and CXCR4 and prevents de novo HIV-1 infection in susceptible cells (Pérez et al., 2010).
References:
Alkon D, Epstein H, Kuzirian A, Bennett M and Nelson T. (2005). Protein synthesis required for long-term memory is induced by PKC activation on days before associative learning. Proc Natl Acad Sci USA. 102:16432-16437.
Boto W, Brown L, Chrest J and Adler W. (1991). Distinct modulatory effects of bryostatin 1 and staurosporine on the biosynthesis and expression of the HIV receptor protein (CD4) by T cells. Cell Regul. 2, 95-103.
Castor T. (1995,1997). Method and Apparatus for Extracting Taxol from Source Materials. US Patent No. 5,440,055 and European Patent No. 689,537.
Castor T. (1998). Method and Apparatus for Isolating Therapeutic Compositions from Source Materials. US Patent No. 5,750,709.
Castor T. (2001). Supercritical Fluid Isolation of Bryostatin 1. SBIR Phase II Final Report. SBIR Grant No. 5 R44 CA 64017-03.
Clamp A and Jayson G. (2002). The clinical development of the bryostatins. Anticancer Drugs. Aug;13(7):673-83.
Davidson S, Allen S, Lim G, Anderson C and Haygood M. (2001). Evidence for the Biosynthesis of Bryostatins by the Bacterial Symbiont “Candidatus Endobugula sertula” of the Bryozoan Bugula neritina. Appl. Environ. Microbiol. 67, 4531-4537.
Do Y, Hegde V, Nagarkatti P and Nagarkatti M. (2004). Bryostatin 1 enhances the maturation and antigen-presenting ability of murine and human dendritic cells. Cancer Res. 64, 6756-6765.
Etcheberrigaray R, Tan M, Dewachter I, Kuipéri C, Van der Auwera I, Wera S, Qiao L, Bank B, Nelson T, Kozikowski A, Van Leuven F and Alkon D. (2004). Therapeutic effects of PKC activators in Alzheimer’s disease transgenic mice. Proc Natl Acad Sci USA. 101:11141-11146.
Favit A, Grimaldi T, Nelson T and Alkon D. (1998). Alzheimer’s-specific effects of soluble b-amyloid on protein kinase C-a and -g degradation in human fibroblasts. Proc Natl Acad Sci USA. 95, 5562–5567.
Hennings H, Blumberg P, Pettit G, Herald C, Shores R and Yuspa S. (1987). Bryostatin 1, an activator of protein kinase C, inhibits tumor promotion by phorbol esters in SENCAR mouse skin. Carcinogenesis. 8:1343–1346.
Mutter R and Wills M. (2000). Chemistry and clinical biology of the bryostatins. Bioorg Med Chem. 8:1841–1860.
Newman D. (1995). Bryostatin: From Bryozoan to Cancer Drug. Presented at the 10th International Bryozoan Meeting, New Zealand.
Olds J and Alkon D. (1993). Protein kinase C: a nexus in the biochemical events that underlie associative learning. Acta Neurobiol. Exp. 53, 197–207.
Pagliaro L, Daliani D, Amato R, Tu S, Jones D, Smith T, Logothetis C and Millikan R. (2000). A Phase II Trial of Bryostatin 1 for Patients with Metastatic Renal Cell Carcinoma. Cancer. Vol. 89, No. 3, 615-618.
Pérez, M, de Vinuesa A, Sanchez-Duffhues G, Marquez M, Bellido M, Muñoz-Fernandez M, Moreno S, Castor T, Calzado M and Muñoz E. (2010). Bryostatin 1 synergizes with histone deacetylase inhibitors to reactivate HIV-1 from latency. Curr HIV Res. 8(6):418-29.
Pettit G, Herald C, Doubek D, Herald D, Arnold E and Clardy J. (1982). Isolation and structure of Bryostatin 1. J. Am. Chem. Soc. 104, 6846–6848.
Philip P, Rea D, Thavasu P, Carmichael J, Stuart N, Rockett H, Talbot D, Ganesan T, Pettit G, Balkwill F and Harris A. (1993) Phase I Study of Bryostatin 1: Assessment of Interleukin 6 and Tumor Necrosis Factor α Induction In Vivo. Journal of the National Cancer Institute. Vol. 85, No. 22.
Philip P and Zonder J. (1999). Pharmacology and clinical experience with Bryostatin 1: a novel anticancer drug. Expert Opin Investig Drugs. 8:2189–2199.
Prendiville J, Crowther D, Thatcher N, Woll P, Fox B, McGown A, Testa N, Stern P, McDermott R, Potter M and Pettit G. (1993). A Phase I Study of Intravenous Bryostatin 1 in Patients with Advanced Cancer. British Journal of Cancer. 68, 418-424.
Qatsha K, Rudolph C, Marmé D, Schächtele C and May W. (1993). Go 6976, a selective inhibitor of protein kinase C, is a potent antagonist of human immunodeficiency virus 1 induction from latent/low-level- producing reservoir cells in vitro. Proc Natl Acad Sci USA. 90, 4674-4678.
Schaufelberger D, Koleck M, Beutler J, Vatakis A, Alvarado A, Andrews P, Marzo L, Muschik G, Roach J, Ross J, Lebherz W, Reeves M, Eberwein R, Rodgers L, Testerman R, Snader K and Forenza S. (1991). The large scale isolation of Bryostatin 1 from Bugula neritina following good manufacturing practices. Journal of Natural Products. 54, 1265-1270.
Suffness M, Newman D and Snader K. (1989). Discovery and Development of Antineoplastic Agents from Natural Sources. Bioorganic Marine Chemistry, Volume 3, Springer-Verlag, Berlin, pp 131-168.
Trenn G, Pettit G, Takayama H, Hu-Li J, Sitkovsky M. (1998). Immunomodulating properties of a novel series of protein kinase C activators. The bryostatins. J. Immunol. 140: 433-9.
Varterasian M, Mohammad R, Shurafa M, Hulburd K, Pemberton P, Rodriguez D, Spadoni V, Eilender D, Murgo A, Wall N, Dan M and Al-Katib A. (2000). Phase II Trial of Bryostatin 1 in Patients with Relapsed Low-Grade Non-Hodgkin’s Lymphoma and Chronic Lymphocytic Leukemia. Clin Cancer Res. 6:825–828.
Vlach J and Pitha P. (1992). Activation of human immunodeficiency virus type 1 provirus in T-cells and macrophages is associated with induction of inducer-specific NF-kappa B binding proteins. Virology. 187, 63- 72.