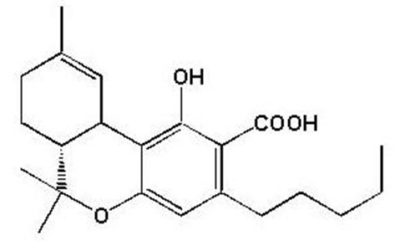
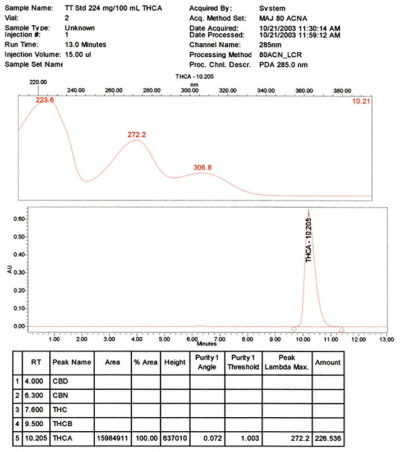
The non-psychoactive precursor of the primary active constituent of marijuana (Cannabis sativa)
- Catalog No: APH-02022
- CAS Number: 23978-85-0
- Chemical Formula: C22H30O4
- Molecular Weight: 358.48
- Purity: > 99% CP HPLC
- Appearance: Slightly colored ethanolic solution
- Solubility: Soluble in methanol and ethanol
- Stability: Δ9-THCA is very unstable, decomposing rapidly in the presence of oxygen, light and acids. It is stable for 3 months at -20°C, 2 weeks at 5°C, and 4 days at room temperature (Zoller et. al. 2000). Δ9-THCA powder appears to be more stable but stability is indeterminate at this time.
- Storage: -20°C
- Shipping: On ice (5°C)
- Handling: Avoid exposure to oxygen, acids and direct sunlight.
Source:
While Δ9-THCA can be synthesized, the process is complex and expensive. Δ9-THCA is highly unstable, decarboxylating readily to form Δ9-THC in air at room temperature. However, in vivo, Δ9-THCA is very stable. In fresh plant material, about 90% of the Δ9-THC obtained from Cannabis sativa is available as Δ9-THCA (Jung et al., 2009).At Aphios, Δ9-THCA is extracted from the flowers and buds of Cannabis sativa(marijuana) utilizing patented SuperFluids™ CXP technology [Castor, US Patent] followed by segmentation chromatography to complete the purification of natural pharmaceutical grade Δ9-THCA for medical use.
Biological Properties:
Δ9-THCA is the biological precursor to Δ9-THC, the primary active constituent responsible for the psychoactive and medicinal properties of marijuana. However, Δ9-THCA itself is not psychoactive. During drying or under intense heat, Δ9-THCA is decarboxylated to form Δ9-THC (Starks, 1977).
A select number of the 65 cannabinoids, including Δ9-THCA, the medically active components of Cannabis, hold the promise of providing new drugs for a variety of treatment purposes. The use of Cannabis for medical treatments has the legal disadvantage that distribution of the plant material to patients violates US Federal law. Smoking Cannabis has the medical disadvantage that the smoke contains many toxic components in common with tobacco smoke, and delivers a highly variable dose of Δ9-THC and other cannabinoids.
Δ9-THCA possesses anti-inflammatory and anti-neurodegenerative properties. Δ9-THCA inhibits cyclooxygenase 1 (COX-1) and cyclooxygenase 2 (COX-2), in human colon cell cultures (Ruhaak et. al., 2011). COX-1 and COX-2 are both enzymes involved in inflammation. Δ9-THCA has also been shown to decrease the amount of oxidative stress caused by mitochondrial impairment, a major mechanism in neural degeneration, in mouse mesencephalic cell cultures (Moldzio et al., 2012).
Natural cannabinoid products directly isolated from the plant Cannabis sativa are being developed for treating pain in cancer patients, cachexia or wasting in AIDS patients and relief to Alzheimer’s patients while increasing their appetite.
There are also currently very high levels of activity in the clinical evaluation of cannabinoid active ingredients and derivatives. In fact, many theoretical and early stage clinical opportunities for cannabinoid therapy are well developed, with a wide range of indications previously or currently in clinical evaluation. Even though Δ9-THCA itself is not currently under any clinical investigations in the United States, its decarboxylated descendant, Δ9-THC, is.
 Marijuana is the most popular and widely used illicit drug among young adults. More than half of US young adults will experiment with Cannabis. The National Survey on Drug Abuse and Health reports that more than 100 million of the US population age 12 and older have tried marijuana at least once.
Marijuana is the most popular and widely used illicit drug among young adults. More than half of US young adults will experiment with Cannabis. The National Survey on Drug Abuse and Health reports that more than 100 million of the US population age 12 and older have tried marijuana at least once.
Although most young adults use Cannabis occasionally and for recreational purpose, some use Cannabis repeatedly and for prolonged periods of time. The Drug Abuse Warning Network estimates that more than 215,000 emergency visits each year are due to marijuana use. NIDA estimates that around 300,000 people entering drug treatment programs reported marijuana as their primary drug of abuse.
References:
Joy JE, Watson SJ and Benson, JA (Eds.). (1999). Institute of Medicine. Cannabis and Medicine: Assessing the Science Base. National Acad. of Sciences: Washington, DC. March 17.
Zoller O, Rhyn P, Zimmerli B. (2000). High-performance liquid chromatographic determination of Δ9-tetrahydrocannabinol and the corresponding acid in hemp containing foods with special regard to the fluorescence properties of Δ9–tetrahydrocannabinol. Journal of Chromatography A. 101-110.
Jung J, Meyer MR, Maurer HH, Neusüss C, Weinmann W, Auwärter V. (2009). Studies on the metabolism of the Delta9-tetrahydrocannabinol precursor Delta9-tetrahydrocannabinolic acid A (Delta9-THCA-A) in rat using LC-MS/MS, LC-QTOF MS and GC-MS techniques. Journal of Mass Spectrometry. 1423-1433.
Starks M. (1977). Marijuana Chemistry: Genetics, Processing and Potency. Ronin. Berkeley, Ca.
Ruhaak LR, Felth J, Karlsson PC, Rafter JJ, Verpoorte R and Bohlin L. (2011). Evaluation of the Cyclooxygenase Inhibiting Effects of Six Major Cannabinoids Isolated from Cannabis sativa. Biological & pharmaceutical bulletin. 774-778.
Moldzio R, Pacher T, Krewenka C, Kranner B, Novak J, Duvigneau JC, Rausch WD. (2012). Effects of cannabinoids Δ(9)-tetrahydrocannabinol, Δ(9)-tetrahydrocannabinolic acid and cannabidiol in MPP+ affected murine mesencephalic cultures. Phytomedicine. 819-824.
National Institutes of Health. (1997). Workshop on the Medical Utility of Cannabis. Report to the Director of NIH by the Ad Hoc Group of Experts. February 19-20.